Enzyme Kinetics Review
Enzyme Kinetics Review. Sections 2.5 to 2.5.3 and 2.7, 2.8 of mikkelsen and cortón, bioanalytical chemistry Atri et al., 2015) and has been the focus of more technically detailed reviews (hansen et al., 2016;

 Enzyme Lineweaver Burk Plot Explained Enzyme from in.pinterest.com
Enzyme Lineweaver Burk Plot Explained Enzyme from in.pinterest.comThe reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Enzyme kinetics is the study of the binding affinities of substrates and inhibitors and the maximal catalytic rates that can be achieved. Review questions for enzyme kinetics:

As a results, the rate of the reaction of an enzyme increases. Elsevier's integrated review pharmacology (second edition), 2012.

Enzyme kinetics is the study of enzyme reactions rates and the conditions which affect them. Under normal conditions, an enzyme with a v

Annual review of biophysics and biomolecular structure regulation of enzyme activity d e atkinson annual review of biochemistry q uantum m echanical m ethods for e nzyme k inetics jiali gao and and donald g. Cleland department of biochemistry, university of wisconsin madison, wisconsin over the past few years the field of enzyme kinetics has undergone conâ siderable growth, accompanied by the development of adequate models for real enzymes, and a true understanding of the principles.

Sections 2.5 to 2.5.3 and 2.7, 2.8 of mikkelsen and cortón, bioanalytical chemistry Thus, a thorough understanding of enzyme kinetics, which dictates the urea hydrolysis rate that is proportional to the rate of caco 3 precipitation in ideal conditions is required for developing an effective framework for caco 3 precipitation in eicp.

The kinetic energy of the enzyme increase when temperature increases, thus will increase the number of collisions of the enzyme and substrate per unit time. Under normal conditions, an enzyme with a v

The kinetic energy of the enzyme increase when temperature increases, thus will increase the number of collisions of the enzyme and substrate per unit time. Product vs time for increasing substrate concentrations initial velocity vs substrate conc.

Elsevier's integrated review pharmacology (second edition), 2012. The kinetic energy of the enzyme increase when temperature increases, thus will increase the number of collisions of the enzyme and substrate per unit time.

For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed reaction (figure 1). As a results, the rate of the reaction of an enzyme increases.

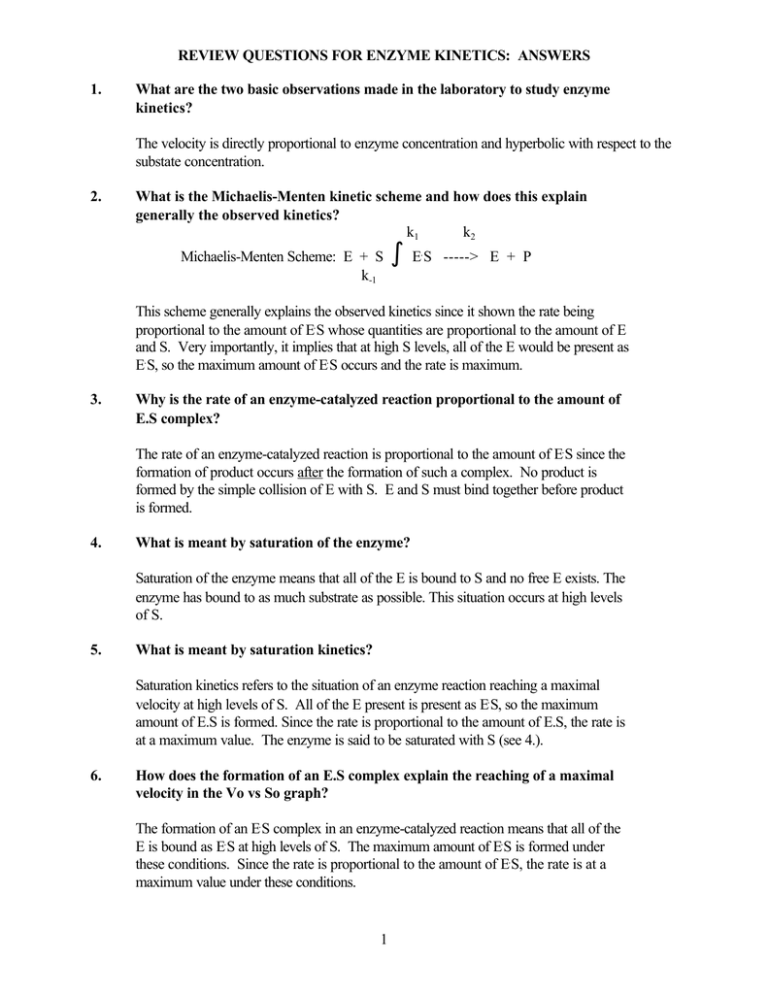
The velocity is directly proportional to enzyme concentration and hyperbolic with respect to the substate concentration. Enzyme active site is saturated.

Following a brief review of methods for fitting steady state kinetic data, modern. Powered by create your own unique website with customizable templates.

1/ v o 1/[s] inhibition issue: Annual review of biophysics and biomolecular structure regulation of enzyme activity d e atkinson annual review of biochemistry q uantum m echanical m ethods for e nzyme k inetics jiali gao and and donald g.
Following a brief review of methods for fitting steady state kinetic data, modern. This book starts with a review of the tools and techniques used in kinetic analysis, followed by a short chapter entitled "how do enzymes work?", embodying the philosophy of the book.
Multisubstrate, immobilized, interfacial, and allosteric The reaction rate is measured and the effects of varying the conditions of the reaction are investigated.

Enzyme kinetics is the study of enzyme reactions rates and the conditions which affect them. Multisubstrate, immobilized, interfacial, and allosteric

What are the two basic observations made in the laboratory to study enzyme kinetics? The velocity is directly proportional to enzyme concentration and hyperbolic with respect to the substate concentration.
Cleland department of biochemistry, university of wisconsin madison, wisconsin over the past few years the field of enzyme kinetics has undergone conâ siderable growth, accompanied by the development of adequate models for real enzymes, and a true understanding of the principles. Shvartsman1,2,4,* akey step towards achemical picture of enzyme catalysis was taken in 1913, when leonor michaelis and maud menten published their studies of sucrose hydrolysis by invertase.

On the right, no saturation is observed and the rate continues to be proportional to the concentration of substrate ([s]). Multisubstrate, immobilized, interfacial, and allosteric

As a results, the rate of the reaction of an enzyme increases. This article offers a comprehensive review of the properties, molecular structure and kinetic mechanisms of urease enzymes.

The reaction rate is measured and the effects of varying the conditions of the reaction are investigated. What substrate concentration is necessary for the enzyme to turn over products at 75% of its theoretical maximum velocity?

The study of enzyme kinetics has been briefly described in several surveys of the itc field (freyer and lewis, 2008; Kinetics are still commonly used, not only in the in vitro context of enzyme characterization but also as a rate law for enzymatic reactions in larger biochemical reaction networks.
The Velocity Is Directly Proportional To Enzyme Concentration And Hyperbolic With Respect To The Substate Concentration.• this model explain how an enzyme can cause kinetic rate enhancement of a reaction and why the rate of a reaction depends on the concentration of enzyme present. What substrate concentration is necessary for the enzyme to turn over products at 75% of its theoretical maximum velocity? Enzyme active site is saturated.
As A Results, The Rate Of The Reaction Of An Enzyme Increases.In this article, we will discuss the structure and function of enzymes, their clinical significance and theories of enzyme kinetics. Elsevier's integrated review pharmacology (second edition), 2012. Review questions for enzyme kinetics:
Formal Laboratory Report On Enzyme Kinetics 3 .However, at high [s], the On the right, no saturation is observed and the rate continues to be proportional to the concentration of substrate ([s]). Cleland department of biochemistry, university of wisconsin madison, wisconsin over the past few years the field of enzyme kinetics has undergone conâ siderable growth, accompanied by the development of adequate models for real enzymes, and a true understanding of the principles.
Enzyme Kinetics Is The Study Of The Binding Affinities Of Substrates And Inhibitors And The Maximal Catalytic Rates That Can Be Achieved.This book starts with a review of the tools and techniques used in kinetic analysis, followed by a short chapter entitled "how do enzymes work?", embodying the philosophy of the book. Here, we discuss how itc can be applied to a broad array of problems in enzyme biochemistry, including. Atri et al., 2015) and has been the focus of more technically detailed reviews (hansen et al., 2016;
Enzyme Kinetic Constants In Vivo Are Largely Unknown, Which Limits The Construction Of Large Metabolic Models.This review aims to give a broad overview of the use of itc to measure enzyme kinetics. Shvartsman1,2,4,* akey step towards achemical picture of enzyme catalysis was taken in 1913, when leonor michaelis and maud menten published their studies of sucrose hydrolysis by invertase. Enzyme kinetics annual review of biochemistry vol.
Belum ada Komentar untuk "Enzyme Kinetics Review"
Posting Komentar